Adaptations will happen in the muscular system if the exercise training emphasises the overload of the muscles aiming to result in hypertrophy and increase in strength.
How does the muscular adaptation happen? I have presented the information in a sequence to help understand how muscular adaptation happens.
First, the muscle fibres are classified into two types in the human body – Type I (slow twitch) and Type II (fast twitch). The type I fibres contract slower compared to the type II fibres.
Next, the muscle fibres contain several proteins and they are:
- Myosin heavy chain (MHC)
- Myosin light chain (MLC)
- Regulatory
- Alkaline (essential)
- Tropomyosin
- Troponin C
- Troponin I
- Troponin T
- Myosin-binding protein C
- Sarcoplasmic reticulum ATPase
Further, the following three techniques can be used to classify the muscle fibres into different sub-types:
- myofibrillar ATPase histochemistry staining ⇒ Type I, IIa, IIx / IIb
- MHC isoform identification ⇒ MHC I, MHC IIa, MHC IIx / MHC IIb
- biochemical identification of metabolic enzymes ⇒ slow twitch oxidative, fast twitch oxidative, fast twitch glycolytic.
The process of muscular adaptation: In the literature, it is mentioned that muscle fibres exist either as pure (only one type of MHC) or as hybrids (1). In most cases, the presence of a particular MHC in a muscle fibre is mirrored by the presence of corresponding mRNA transcript. However, following endurance training and during the detraining period, the muscle fibres will be in the process of changing the expression of their MHCs. In these fibres, it was found that MHC mRNA transcripts do not match the corresponding protein (primarily in IIa and IIx fibres) (2). Anderson et al (2) propose that these fibres represent transitional fibres giving us a clue to the direction of change in MHC gene expression. The authors (2) confirm that such adaptation was noticed more frequently in biopsies obtained after a training or detraining period than before the training period.
Factors influencing MHC expression: Staron et al (3) report that MHC expression may vary along the length of some muscle fibres. Different segments of the same muscle fibre may alter their proteins at different times depending on certain factors like (1):
- the distance of the fibre segment from the nerve terminal,
- the adequacy of the available blood supply and
- the vectors of stress and strain to which the fibre is subjected.
Hence, there is a poor correlation between the MHC composition of a muscle and the muscle’s performance due to varying MHC expression along the length of the muscle fibre. This is an important finding as we now know that vectors of stress and strain elicit training adaptation through MHC expression on specific segments of muscle fibres where load transfer takes place.
How can we measure/quantify training adaptations or characteristics of muscle fibres?
The muscular training adaptations and/or muscle fibre characteristics can be measured by estimating the changes in:
- the proportion of the fibre type,
- the absolute or relative cross-section area of the muscle fibres,
- the shortening velocity of a single muscle fibre,
- the energy cost of contraction of a single muscle fibre.
The changes in the proportion of the fibre type and the cross-sectional area of muscle fibres can be determined by taking samples of muscle fibres through biopsy and by analysing using the above-mentioned staining/histological techniques.
The shortening velocity of a single muscle fibre can be studied using the following techniques (1):
- slack test (measures single fibre unloaded shortening velocity, Vo)
- Vo is a powerful index to determine the intrinsic shortening of sarcomeres than Vmax. [Vo: I < IIa < IIx < IIb]
- force-velocity relationship (single fibre maximal shortening velocity, Vmax)
- Vmax is useful to determine muscle fibre power at different shortening speeds.
- muscle fibre power (Vopt, the optimal velocity at which peak power occurs). [Vopt: I < IIa < IIb]
- specific tetanic tension (maximum tetanic tension per unit cross-section area). [I < IIa < IIx]
The energy cost of contraction of a single muscle fibre can be studied by estimating myofibrillar ATPase activity (i.e., the rate of ATP hydrolysis) and the force-time integral of the contraction (1):
- The energy cost of contraction (of a single muscle fibre) depends on the speed with which the cross-bridges consume ATP during muscle contraction.
- The maximal shortening velocity of a muscle fibre reflects the cross-bridge ATP turnover rate, which is influenced by the enzymatic activity of the myofibrillar ATPase.
- The rate of ATP hydrolysis during a tetanic contraction is highest in type IIx fibres followed by IIa and I fibres (4).
- The myosin ATPase is highest in type IIa and IIx fibres followed by I fibres (5).
- The energy cost for type IIb fibres is four times that of I fibres (4).
- The type II fibres are faster and stronger during a contraction but energetically costlier than type I (slow) fibres.
- As the muscular contraction force increases, it becomes economically less viable (requiring more ATP) and thus, the muscle fibres are recruited in the following order: type I, IIa, IIax and IIx.
Additional points to remember (1):
- Maximum force generated by a single muscle fibre ∝ Number of cross bridges that are arranged in parallel and number of cross bridges that are in force generating phase of cross-bridge cycling at that moment.
- Force generated by a muscle ∝ Number of muscle fibres arranged in parallel, that are activated by the nervous impulse.
- Maximum force potential of a muscle ⇒ determined by the maximum number of muscle fibres arranged in parallel, that a nerve impulse can activate at one instance.
- A muscle can increase its force potential ⇒ when fibres become larger in cross-sectional area, having more cross bridges arranged parallel, which can be in force generating phase of a contraction.
- Force generating capacity of a muscle is a function of its physiologic cross-sectional area (PCSA).
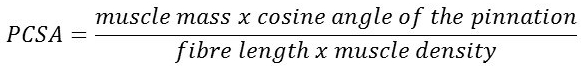
- Upper body has a large proportion of type II fibres and lower body has a large proportion of type I fibres.
- Strength and power athletes have a large proportion of type IIa fibres and endurance athletes have a large proportion of type I fibres.
- Strength training increases the proportion of type I, IIa and IIx fibres and causes fibre proportion shift from type IIx to IIa.
- Endurance training increases mitochondrial enzyme content, alters proteins that control cellular calcium levels, decreases type IIb fibres and increases type IIa and I fibres.
- Type of exercise training may cause fibre type shifts but is more likely to happen if one has genetic preposition (eg., ACTN3 R allele).
References:
- Gardiner, Phillip F. Neuromuscular aspects of physical activity. Human Kinetics, 2001.
- Andersen, Jesper Løvind, and S. T. E. F. A. N. O. Schiaffino. “Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibers.” American Journal of Physiology-Cell Physiology 272.6 (1997): C1881-C1889.
- Staron, Robert S., and Dirk Pette. “Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles.” Pflügers Archiv European Journal of Physiology 409.1 (1987): 67-73.
- Stienen, G. J., et al. “Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence.” The Journal of Physiology 493.Pt 2 (1996): 299.
- Pereira Sant’Ana, J. A. A., et al. “Comparison of the molecular, antigenic and ATPase determinants of fast myosin heavy chains in rat and human: a single-fibre study.” Pflügers Archiv European Journal of Physiology 435.1 (1997): 151-163.