All metabolic processes in the body require energy supply (food) but depend on the use of oxygen (O2) for extraction of energy which ultimately results in heat production.
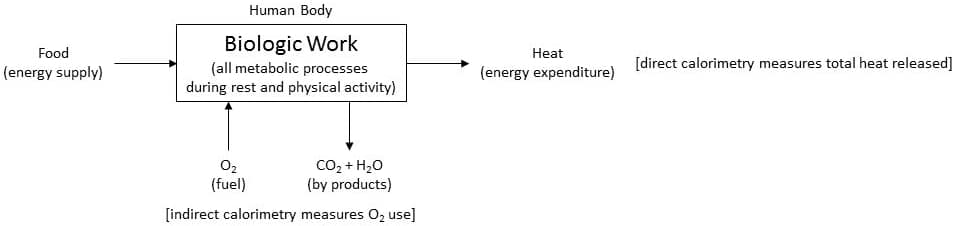
There are two approaches to quantify human energy expenditure during rest and physical activity. They are:
- direct calorimetry and
- indirect calorimetry.
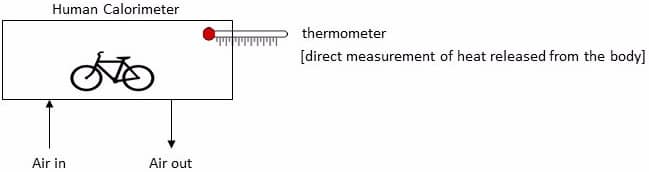
Direct calorimetry is the method of directly quantifying the body’s rate of biologic work (energy expenditure/metabolism) by measuring body’s heat production.
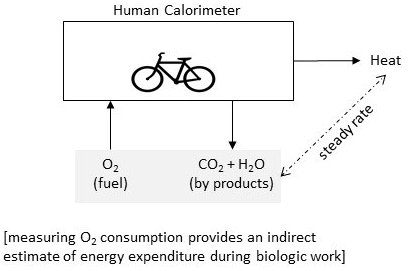
Indirect calorimetry is the method of indirectly quantifying the body’s rate of biologic work by measuring body’s O2 use.
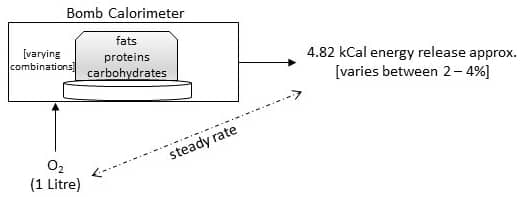
When we say ‘burn the calories’, what do we really mean? Studies show that when we burn a food mixture (varying combinations of proteins, fats, carbohydrates) with one litre of O2 in a bomb calorimeter, there is a release of approximately 4.82 kCal.
The steady rate conditions between O2 consumption and energy release while burning varying mixture of food constituents provide us with a reliable method (measuring O2 uptake) to quantify biologic work during rest and physical activity.
There are two methods to perform indirect calorimetry:
- closed circuit spirometry and
- open circuit spirometry.
In closed circuit spirometry, the subject rebreathes from a spirometer filled with 100% O2. When the subject continues breathing (in and out from the spirometer) for a pre-determined time interval, the amount of O2 in the spirometer reduces as some O2 is used by the subject’s body. The total oxygen consumption can be calculated by subtracting the initial amount of O2 (100%) in the spirometer to the final amount of O2 at the end of the pre-determined time interval.
In open circuit spirometry, the subject breathes ambient air with a constant composition. This method involves measuring the total volume of air breathed during a specified time interval, and measuring the O2 / CO2 concentrations in the expired air during rest or physical activity for the same time interval. The total O2 consumption can be calculated by determining the changes in O2 / CO2 concentrations in expired air compared to ambient air.
The three common techniques of measuring O2 consumption using open circuit spirometry method are:
- classic bag technique,
- portable breath by breath gas analysis technique and
- portable gas analysis technique.
Respiratory Quotient: When we burn food mixtures with varying combinations of proteins, carbohydrates and fats using one litre of O2, there is a release of approximately 4.82 kCal. However, this calorific value of O2 varies between 2 to 4% with large variations in the food constituents. We must account for this variability while determining body’s energy expenditure (heat production) through indirect calorimetry. This requires the measurement of CO2 produced per unit of O2 consumed with all three types of substrates (protein, carbohydrates, fats). It can be expressed as a ratio called respiratory quotient (RQ) for all three substrates.
The respiratory quotient is defined as the ratio of CO2 produced to O2 consumed while food is processed in the body:
RQ = CO2 produced ÷ O2 consumed
1. Carbohydrate metabolism:
The chemical equation for the oxidation of glucose:
C6H12O6 + 6 O2 → 6 CO2+ 6 H2O
RQ = 6 CO2 / 6 O2 = 1.0
2. Fat metabolism:
The chemical equation for the oxidation of palmitic acid is:
C16H32O2 + 23 O2 → 16 CO2 + 16 H2O
RQ = 16 CO2 / 23 O2 = 0.696
3. Protein metabolism:
The chemical equation for the oxidation of albumin is:
C72H112N18O22S + 77 O2 → 63 CO2 + 38 H2O + SO3 + 9 CO(NH2)2
RQ = 63 CO2 / 77O2 = 0.8
4. Non-protein metabolism:
RQ = 2.78 Litres of CO2 / 3.22 Litres of O2 = 0.86
As the food mixtures always have a combination of all three substrates, none of the above RQ can be assigned to the protein metabolism in the diet. A commonly used estimate is 0.8 as it is a metabolism of a mixture of 40% carbohydrates and 60% fats. We can thus apply the caloric equivalent of 4.825 kCal of O2 for energy transformations.
References:
- Péronnet, F. and Massicotte, D., 1991. Table of nonprotein respiratory quotient: an update. Can J Sport Sci, 16(1), pp.23-29.
- Zuntz, N. and Schumburg, W.A.E.F., 1901. Studien zu einer Physiologie des Marsches (Vol. 6). Hirschwald.
- Lusk, G., 1924. Animal calorimetry. Analysis of the oxidation of mixtures of carbohydrate and fat. A correction. J. Bio. Chemi., 59:41-42.
- Richardson, H.B., 1929. The respiratory quotient. Physiological Reviews, 9(1), pp.61-125.
- American College of Sports Medicine, 2013. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins.